Iron Sulfate: What is it and how do we apply it in agriculture?
Iron(II) sulfate is an ionic compound that is also known as ferrous sulfate.
Iron sulfate or ferrous sulfate denotes a range of salts with the formula FeSO4-xH2O.
These compounds commonly exist as the heptahydrate (x = 7) but are known by different values of x. The hydrated form is used medically to treat iron deficiency, and also for industrial and/or agricultural applications.
What is the formula of iron sulfate?
Its chemical formula is: FeSO4, that is, it is made up of iron, sulfur and oxygen.
Caution: Take precautions with iron sulfate
Although iron sulfate is derived from a natural source, it can cause burning eyes and skin. Wear protective gloves, a dust mask, and goggles for safety.
If the product is accidentally ingested, seek medical attention immediately. If there are children, keep it out of their reach.
Prevent iron sulfate solution or dry powder from getting on concrete, brick, or other masonry and/or painted surfaces.
How to know quickly if your plants need iron?
Before testing the soil, there may be some visual clues that your plants are lacking in iron.
Yellow leaves are a common indicator of this problem. Iron chlorosis is caused by iron deficiency.
While there may be other causes of yellow leaves, such as insufficient nitrogen, iron chlorosis is characterized by yellowing beginning during new plant or leaf growth, rather than existing leaves turning yellow.
 Characteristics of iron sulfate
Characteristics of iron sulfate
Most of the time it is found in the form of a salt or as a crystalline solid and is bluish-green or yellow in color with brown tints; however, the most common presentation is blue-green.
Its boiling point is 90°C and its melting point is 64°C if it is in anhydrous form. Its solubility in water at 20°C is 30 grams per litre.
In general, iron (II) sulfate is not flammable; however, as it is a weak reducing agent, it can react with oxidizing agents and generate heat or products that may be flammable or combustible.
But iron-derived salts are not considered to be highly flammable products.
When iron sulfate (II) is in the presence of dry air, it behaves like an efflorescent substance, that is, it loses water causing its crystalline structure to change to one more similar to the crusts created by dust.
While in humid environments it is covered with iron sulfate (III), which gives it a brownish color.
When iron(II) sulfate is mixed with water to form an aqueous solution it has a pH with levels that have to acidity due to a process called hydrolysis.
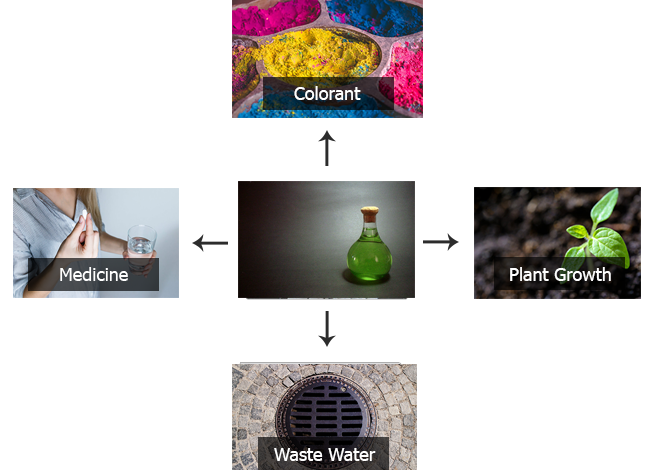
Among its many uses is that of serving as an ingredient to purify water and eliminate phosphates in purification plants.
It is also used to treat anemia; however, remember to check with your doctor before using it for this purpose as it can have side effects such as nausea and stomach pain.
What is iron sulfate used for in agriculture?
One of the most common deficiencies in soils is iron.
This problem occurs in many parts of the world and generates problems that can end up with the harvest or cause deficiencies in the development of the plant and production of the fruit or flower.
In particular, the lack of iron causes problems to synthesize chlorophyll and therefore that the plant performs photosynthesis.
When and how to apply iron sulfate in your garden?
You can use iron sulfate whennotice deficiencies of this nutrient.
Where to get iron sulfate?
Most of the time it comes already packaged in solid presentations. You can get it at any garden store or online.
How do we use it?
Visible damage from lack of iron can vary depending on the plant species; however, the main features are:
- Chlorosis – This is basically a discoloration of the characteristic green of the leaves; instead yellowness appears in the foliage. The most affected leaves are the young ones presenting large yellow spots, while the older ones may only have a lighter green.
- In most plants it begins to be noticed at the top.
- There is a growth retardation.
- In more advanced stages, the leaves begin to burn starting with the contour and extending to the interior.
Analyze the type of soil you have before applying iron sulfate
Soil pH kits are available in many gardening, DIY, agriculture stores… which makes it possible for you to measure the pH level of the garden soil yourself.
Most vegetables and flowers grow best in soil with a pH between 6.0 and 7.0, slightly acidic to neutral, although certain plants, called acidic, grow best when the pH is below 6, in the 5 range..0 to 6.0.
Iron is most readily available to plants when soil pH is below 7, since alkaline soil binds iron particles and plants cannot absorb iron.
If the soil is too alkaline, the application of iron sulfate can lower the pH, but common sulfur is more often used for this purpose.
Why can there be iron deficiency in the soil?
The most common causes for iron deficiency is due to factors such as pH, redox potential and the types of minerals present in the soil.
This often causes it to remain in very low solutions. Likewise, the solubility of Iron also depends on the content of organic matter present in the crop.
extreme temperaturesThey cause the absorption capacity of iron and other nutrients to decrease considerably.
Some studies indicate that the temperatures where the greatest absorption is generated are between 30 and 37°C (however, take into account the requirements of each plant since very high temperatures can cause greater damage).
On the other hand, when temperatures are between 10 and 17°C, absorption can decrease on average two or three times compared to the optimum absorption temperature.
Another aspect that can decrease iron absorption is an increase in other nutrients such as copper, zinc, nickel, cadmium, sodium and potassium (we are talking about a surplus, not an ideal amount).
For this reason, it is important that you do not exceed the amount indicated on the package and do it every so often to allow the plant to absorb the elements little by little.
How is iron sulfate prepared? (7 steps)
- Try to store iron sulfate away from any source of moisture to prevent it from reacting and losing its main functions.
- Store it in a dry place, without direct sunlight and without the presence of heat.
- Also try to ensure that the container where you keep it is well closed.
- To add iron sulfate to crops you first have to take into account the pH levels.
- Iron absorption is greater at high acidity levels while neutral or alkaline absorption is practically nil (values greater than 8). It is recommended that you consult with an expert about the feasibility of adding this compound to the crop, as it may be harmful.
- A highly recommended option to avoid deficiencies of this nutrient in crops is the application of iron chelates. These are applied via the root (to be absorbed by the roots).
- Likewise, there are different brands on the market, so accessing them is usually not a problem. This type of fertilizer is usually 50% more expensive than the others; however, they are usually efficient.
How should we apply iron sulfate?
Apply iron sulfate to your soil or plant foliage according to label directions.
For the product that is sold with 20% iron sulfate, it is recommended for flowers, shrubs and trees, with a dry application of 100-200 grams per 30 square meters, evenly distributed.
Use 1.3 to 2.7 kg of iron sulfate per 30 square meters to lower the soil pH by one unit, using more in heavy soils and less in sandy soils.
Other products, which contain 6% sulfur and 15% iron, dry application of 200 grams per 9 square meters is recommended for flowers and vegetables. For the liquid application of 20% iron sulfate, 200 grams per 4 liters of water is recommended.
The product with 15% concentration is recommended between 100-200 grams per 4 liters of water. Spray the solution on the foliage until it is completely wet.
What crops benefit from it?
It has been used to plant citrus trees or plants (such as lemon), peach, kiwi, rice, tomatoes or any other plant that is deficient in this nutrient.
Normally iron is already added together with other nutrients in fertilizers, but if the problem is serious you can use this compound.

Other uses of iron sulfate
- In the second half of the 1850s iron sulfate was used as a photographic developer for collodion process images.
- Iron sulfate is sometimes added to the cooling water flowing through the brass tubes of turbine condensers to form a protective corrosion-resistant coating.
- It is used in gold refining to extract metallic gold from solutions of auric chloride (gold dissolved in solution with aqua regia).
- It has been used in water purification by flocculation and for the removal of phosphates in municipal and industrial wastewater treatment plants to prevent eutrophication of surface water bodies.
- It is used as a traditional method of treating wood paneling in houses, either alone, dissolved in water, or as a component of water-based paint.
- Green vitriol is also a useful reagent in the identification of fungi.
- It is used as the iron catalyst component of Fenton’s reagent.
- In the early 19th century, the chemist Friedrich Accum discovered that in England the porter of dark beer often contained iron(II) sulfate as a foaming agent.
- It is one of the key ingredients of iron ink.

![Photo of Adiantum: [Cultivation, Substrate, Irrigation, Care, Pests and Diseases]](https://www.complete-gardening.com/wp-content/uploads/2022/08/adiantum-cultivation-substrate-irrigation-care-pests-and-diseases-390x220.jpg)


